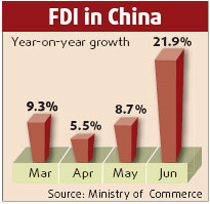
US pharmaceutical firm Merck & Co and its China importer have recalled 104,930 vials of a children's vaccine against meningitis and pneumonia after they were found to be defective, China's top drug watchdog said yesterday.
Hib is a vaccine for under-five children against meningitis, pneumonia and other serious infections.
The vaccines were distributed in eight Chinese municipalities and provinces, including Beijing and Tianjin, and Zhejiang, Fujian, Guangdong, Hainan and Sichuan, said a statement on the State Food and Drug Administration's website (SFDA). But there have been no reports from any of these places of a child falling ill after vaccination.
In fact, Merck has recalled 1.2 million vials of Hib across the world after finding sterilization defects in the lot made at its Pennsylvania factory on Wednesday.
"The SFDA received the recall report from Merck, and immediate action was taken to track down and help recall the products in China," SFDA spokeswoman Yan Jiangying said.
"Medical institutions in all of the eight places have stopped administering the vaccine, and the existing stock has been sealed."
Guangdong Province had bought 26,340 of the vials, 7,120 of which have already been administered to children, the Southern Metropolis Daily said. And 60 of the 2,640 vials distributed in Beijing have been injected, according to Xinhua.
"All provincial disease control centers have issued urgent notices to order the immediate recall of the vaccines," Yan said.
Medical experts have advised parents who had already been administered Hib not to panic because all imported drugs undergo strict tests before entering the country, and would have been banned in the first place had they been found to be harmful.
(China Daily December 17, 2007)